Exercises
Please complete each exercise or answer the question before reviewing the posted solution comments.
Use the clear command before starting your work on a new problem
to ensure that any existing variable name assignments don't interfere with your work on
the new problem.
-
The decay constant (lambda) of an isotope can be calculated by dividing the natural log of 2 by the half-life (t1/2). Specifically, the equation is:
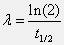
Iodine-131 is used extensively in nuclear medicine because of its short half-life and useful beta decays. Specifically, the half-life of I-131 is 8.04 days. Use the command line window and Matlab's built-in functions to solve for the decay constant (in [days-1]).
0.0862days-1 Note: Matlab does not have a built-inln()function. The built-in functionlog()function is the natural logarithm. -
Note: Please complete Question 1 before attempting Question 2. The activity of a radioactive substance is the rate at which decays occur in the sample. Further, the activity can be expressed by the equation:
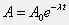
A radioactive sample has an activity of 4.5 uCi (1 Ci is 3.7E10 disintegrations/second). The half-life of the substance is 68 s. Use Matlab's built-in functions and the command line window to calculate the activity (in uCi) of the substance at t=60 s.
2.4412 uCiNote: Use the built-inlog()function andexp()function in this exercise.